

30 March, 2020
Gengwu Zhang, Abdul-Hamid Emwas, Umar F. Shahul Hameed, Stefan T. Arold, Peng Yang, Aiping Chen, Jun-Feng Xiang, Niveen M. Khashab; Shape-Induced Selective Separation of Ortho-substituted Benzene Isomers Enabled by Cucurbit[7]uril Host Macrocycles; Chem, March 30, 2020
Abstract
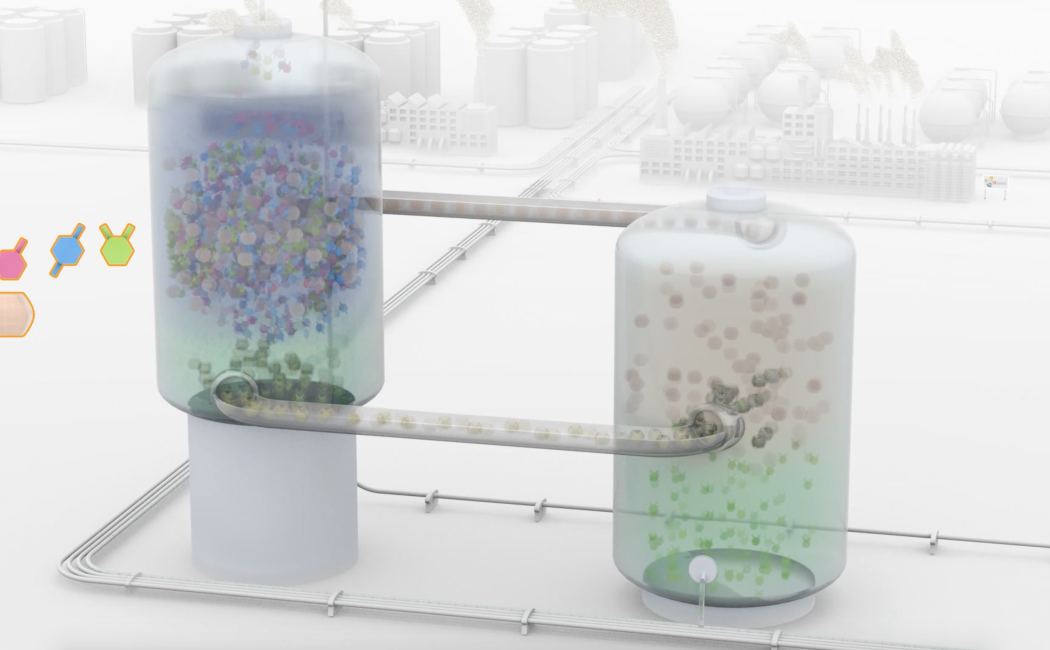
The separation of benzene derivatives is energy intensive and laborious as a result of the overlapping physicochemical properties of these isomers. Here, we report on the separation of ortho-disubstituted benzene isomers using cucurbit[7]uril (CB7) aqueous solution with more than 92% selectivity. Thermodynamic and kinetic analysis proves that the ortho-isomer has stronger binding ability and slower decomplexation rate constant than the para- and meta-isomers when hosted by CB7. Optimized host-guest models indicate that the ortho-isomer with the smallest aspect ratio well matches the spherical interior cavity of CB7, resulting in highly stable complexes. Furthermore, laboratory scale-up experiments using commercial xylenes and C8 aromatic fraction of pyrolysis gasoline proved that CB7 is able to separate ortho-xylene (OX) with a remarkable selectivity of up to 83%. We believe that this work accentuates the role of molecular recognition studies using macrocyclic hosts to improve the quality and energy bill of critical industrial separations.
