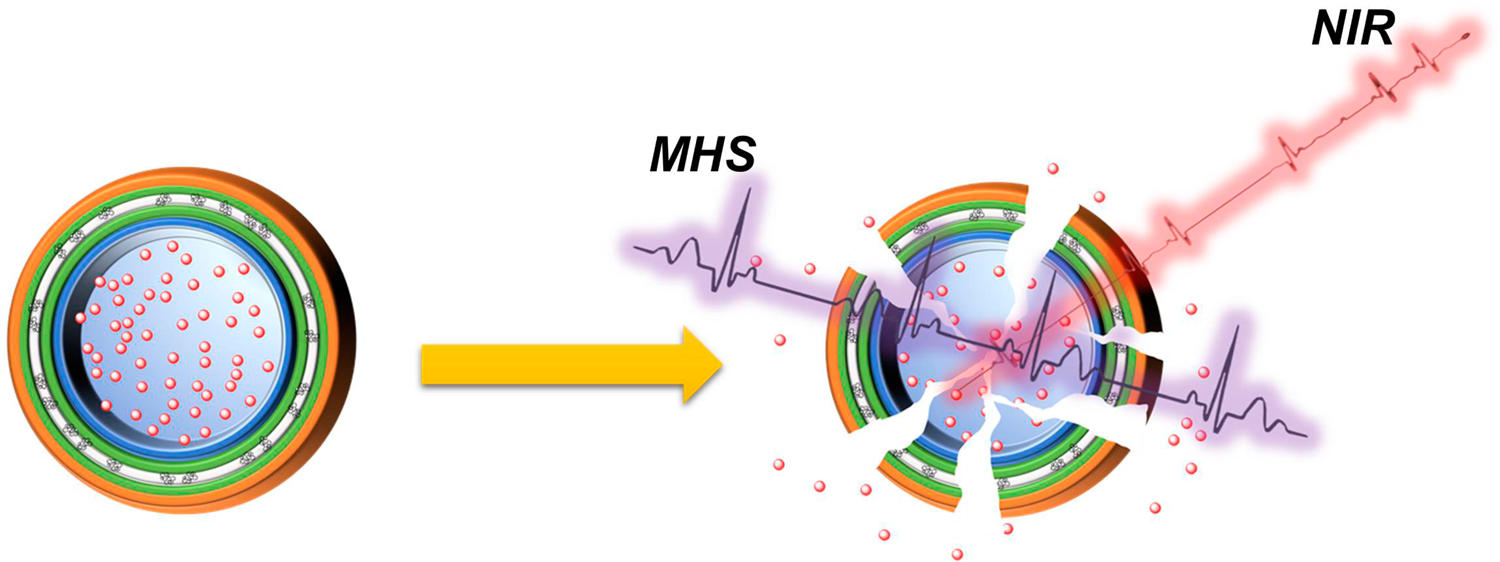
Premature drug release is a common drawback in stimuli responsive drug delivery systems (DDS) especially if it depends on internal triggers, that are hard to control, or a single external stimulus, that can only have one function. Thus, many DDS systems were reported combining different triggers, however limited success has been established in fine-tuning the release process mainly due to the poor bioavailability and complexity of the reported designs. This paper reports the design of a hybrid microcapsule (h-MC) by a simple layer-by-layer technique comprising polysaccharides (Alg, Chi, HA), iron oxide, and graphene oxide. Electrostatic assembly of the oppositely charged polysaccharides and graphene sheets provided a robust structure to load drugs through pH control. The polysaccharides component ensured high biocompatibility, bioavailability, and tumor cells targeting. Magnetic field and near infrared laser triggerable Fe3O4@GO component provided dual high energy and high penetration hyperthermia therapy. On-demand drug release from h-MC can be achieved by synchronizing these external triggers, making it highly controllable. The synergistic effect of hyperthermia and chemotherapy was successfully confirmed in vitro and in vivo.