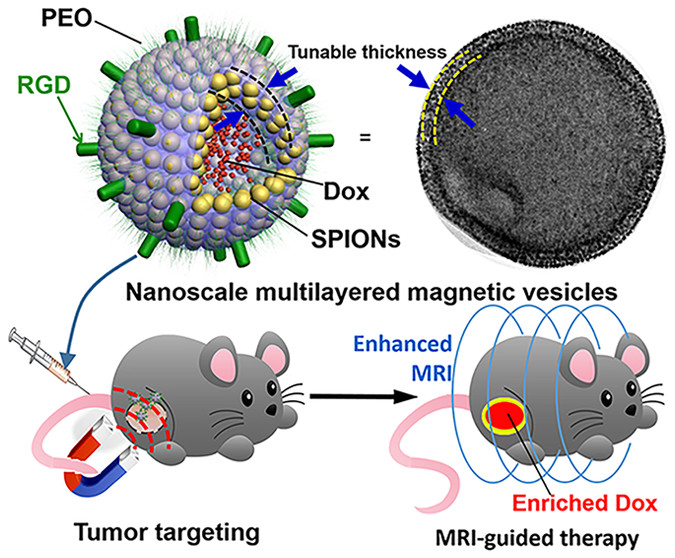
This article describes the fabrication of nanosized magneto-vesicles (MVs) comprising tunable layers of densely packed superparamagnetic iron oxide nanoparticles (SPIONs) in membranes via cooperative assembly of polymer-tethered SPIONs and free poly(styrene)- b-poly(acrylic acid) (PS- b-PAA). The membrane thickness of MVs could be well controlled from 9.8 to 93.2 nm by varying the weight ratio of PS- b-PAA to SPIONs. The increase in membrane thickness was accompanied by the transition from monolayer MVs, to double-layered MVs and to multilayered MVs (MuMVs). This can be attributed to the variation in the hydrophobic/hydrophilic balance of polymer-grafted SPIONs upon the insertion and binding of PS- b-PAA onto the surface of nanoparticles. Therapeutic agents can be efficiently encapsulated in the hollow cavity of MVs and the release of payload can be tuned by varying the membrane thickness of nanovesicles. Due to the high packing density of SPIONs, the MuMVs showed the highest magnetization and transverse relaxivity rate ( r2) in magnetic resonance imaging (MRI) among these MVs and individual SPIONs. Upon intravenous injection, doxorubicin-loaded MuMVs conjugated with RGD peptides could be effectively enriched at tumor sites due to synergetic effect of magnetic and active targeting. As a result, they exhibited drastically enhanced signal in MRI, improved tumor delivery efficiency of drugs as well as enhanced antitumor efficacy, compared with groups with only magnetic or active targeting strategy. The unique nanoplatform may find applications in effective disease control by delivering imaging and therapy to organs/tissues that are not readily accessible by conventional delivery vehicles.