

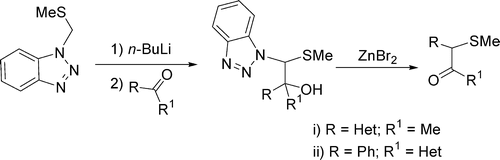
The anion formed from the lithiation of 1-[(methylthio)methyl]-1H-benzotriazole 1 with n-BuLi adds to heteroaryl ketones to give 2-benzotriazolyl alcohols 3a-m. Thermolysis of 3a-g in the presence of zinc bromide induces a 1,2-shift of heteroaromatic groups to form ketones 4a-g. By contrast, in the rearrangement of 2-benzotriazolyl alcohols 3h,i,k-m migration of the phenyl group rather than the corresponding heteroaromatic groups occurred to give ketones 4h,i,k-m.